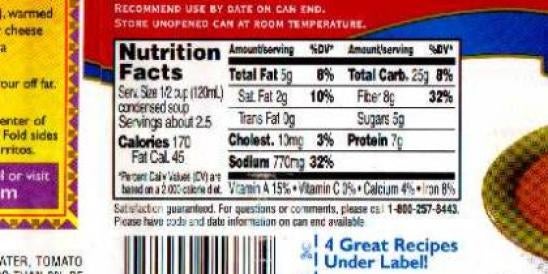
The U.S. Food and Drug Administration (FDA) announced additional changes to its proposal to revise the requirements for nutritional labeling and is separately reopening the comment period for its proposed nutrition labeling changes. The new proposed changes and the reopening of the comment period specifically deal with FDA’s Nutrition Facts and Supplement Facts labels. FDA announced both of these changes in today’s issue of the Federal Register (80 Fed. Reg. 44,302 and 44,303, July 27, 2015).
The changes to the proposed rule include requiring a declaration of the percent daily value (%DV) for added sugars. The rule proposal is a supplement to FDA’s March 3, 2014 proposed rule that, among other things, mandated “added sugars” be listed on the nutritional label (we have previously published an article on that proposed rule). Added sugars do not include those occurring in foods before they are processed or packaged.
FDA is proposing that the daily value for added sugars will be based on the recommendation from the 2015 Dietary Guidelines Advisory Committee that calories from added sugars should not exceed 10% of a daily diet. Based on the nutritional label’s current assumption of a 2000 calorie diet, a 10% limit would mean a typical 20 oz. soda would provide more than 100% of the DV for added sugar. To help consumers better understand the DV information, the proposed rule also changes the DV explanatory footnote on the nutritional label to read:
* The percent daily value (%DV) tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice.
The FDA has also reopened the comment period on its original proposed rule to invite comments on two consumer studies conducted by FDA. The first study examined consumer eye-tracking to determine the potential effects of several possible changes to the Nutrition Facts label on consumer viewing and use of the label. The second study was a web-based experiment to explore whether modifications to the format of the Nutrition Facts label would affect consumers’ interpretation of information presented in the label.
This announcement moves any potential for final action on FDA’s changes to the Nutrition and Supplement Facts labels to next year. Comments on the changes to the percent daily value footnote and percent daily value for sugars are due by October 13, 2015, and may be submitted electronically at this link, while comments on FDA’s consumer studies are due September 25, 2015, and may be submitted at this link.





 i
i


