Policy Update-CMS Releases FY 2022 IPPS Final Rule
On August 2, 2021, the Centers for Medicare and Medicaid Services (CMS) posted the FY 2022 Inpatient Prospective Payment System (IPPS) final rule. Effective October 1, 2021, the final rule updates Medicare payment policies and quality reporting programs relevant for inpatient hospitals, and seeks to address challenges related to the COVID-19 pandemic.
CMS stated that the FY 2022 IPPS final rule will be issued in multiple parts. The agency will address comments on proposals related to disproportionate share hospital payments, organ acquisition costs, and the Consolidated Appropriations Act, 2021 (CAA) provision concerning payments to hospitals for direct graduate medical education (GME) and indirect medical education in subsequent publications.
The final rule is available here. A CMS factsheet on the final rule is available here. The final rule is scheduled to be published in the Federal Register on August 13, 2021.
Key Takeaways
-
CMS estimates that the payment update and other rule changes will increase IPPS payments to hospitals in FY 2022 by approximately $2.3 billion. This estimate does not factor in changes in hospital admissions, real case-mix intensity or the mandatory sequestration adjustment.
-
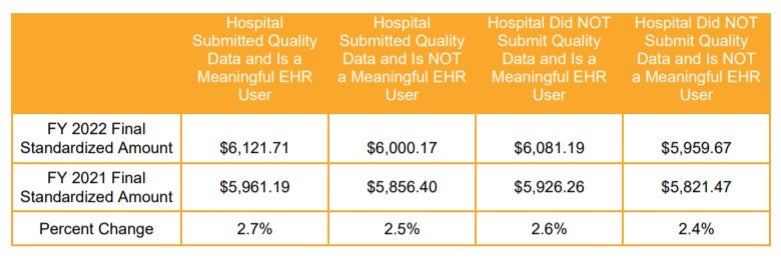
The FY 2022 standardized amount for hospitals that successfully participate in the Hospital Inpatient Quality Reporting (IQR) Program and that are meaningful electronic health record (EHR) users will be $6,121.71, an increase of 2.7% compared to the final FY 2021 standardized amount.
-
CMS will use FY 2019 Medicare Provider Analysis and Review (MedPAR) data for the FY 2022 ratesetting process given the impact of the COVID-19 public health emergency (PHE) on inpatient utilization and case mix in FY 2020.
-
As a one-time exception, also because of pandemic impacts, CMS will provide a one-year extension for 13 technologies whose New Technology Add-on Payment (NTAP) period was scheduled to expire.
-
Hospitals will receive neutral payment adjustments under the Hospital Value-Based Purchasing (HVBP) Program for FY 2022.
-
CMS will address its proposal related to the CAA, including distribution of 1,000 new Medicarefunded medical residency positions, in subsequent parts of the final rule.
-
CMS outlines comments it received on its requests for information (RFIs) related to digital quality mcdermottplus.com 2 measures and health equity, noting that it will make changes through separate and future noticeand-comment rulemaking as necessary
Standardized Amount
Key Takeaway: CMS finalized a payment increase of 2.7% for hospitals that successfully participate in reporting programs.
The standardized amount is the dollar-based base unit used to determine payments to hospitals for inpatient services furnished to Medicare beneficiaries. Each year, CMS updates the standardized amount for inflation based on the hospital market basket index, then applies a variety of other statutorily mandated or inspired adjustments.
The standardized amount varies based on an individual hospital’s participation in the Hospital IQR and EHR meaningful use programs. The FY 2022 standardized amount for hospitals that successfully participate in both programs is $6,121.71. This represents an increase of 2.7% over the final FY 2021 standardized amount ($5,961.19).
The 2.7% increase to the standardized amount reflects a 2.7 percentage market basket update, less a 0.7 percentage point productivity adjustment, plus a 0.5 percentage point positive adjustment for documentation and coding mandated by Section 414 of the Medicare Access and CHIP Reauthorization Act of 2015 for FY 2018 through FY 2023. In addition to these statutory updates, the standardized amount is subject to budget neutrality adjustments discussed in the final rule. Hospitals that fail to submit quality data are subject to a -0.675% adjustment, and hospitals that fail to be meaningful EHR users are subject to a -2.025% adjustment.
FY 2022 standardized amounts, shown in the table below, are the sum of the labor-related and nonlabor-related shares without adjustment for geographic factors. The labor-related share reflects the proportion of the federal base payment that is adjusted by a hospital’s wage index. For FY 2022, CMS finalized as proposed the downward revision of labor-related share from 68.3% to 67.6%. This change is the result of a statutory requirement and should have limited impact on FY 2022 and future IPPS payment for most hospitals
Market-Based MS-DRG Relative Weight Methodology and Data Collection
Change in Methodology for Calculating MS-DRG Relative Weights
Key Takeaway: CMS repealed the policy that would use market-based data for calculating Medicare Severity Diagnosis Related Group (MS-DRG) relative weights, including its requirement to report Medicare Advantage data.
CMS calculates payment for a specific case under the IPPS by multiplying an individual hospital’s geographically adjusted standardized amount per case by the relative weight for the MS-DRG to which the case is assigned. Each MS-DRG relative weight represents the average resources required to care for cases in that particular MS-DRG, relative to the average resources required to care for cases across all MS-DRGs. MS-DRG classifications and relative weights must be adjusted at least annually to account for changes in resource consumption.
In the FY 2021 IPPS rulemaking cycle, CMS finalized a new market-based methodology for estimating MSDRG relative weights based on median payer-specific negotiated charge information collected on Medicare cost reports. This new methodology was scheduled to begin in FY 2024 without any phase-in period. It also was very controversial.
CMS has now reversed course, electing not to pursue this policy. CMS will instead maintain its existing methodology for determining MS-DRG weights for FY 2024 and beyond.
The agency also finalized a proposal to repeal the corresponding requirement that hospitals report on the Medicare cost report, ending on or after January 1, 2021, the median payer-specific negotiated charge by MS-DRG that a hospital has negotiated with all of its Medicare Advantage payers.
MS-DRG Changes
Key Takeaway: CMS finalized its proposal to use FY 2019 MedPAR data and the FY 2018 Healthcare Cost Report Information System file for analyzing MS-DRG changes and determining MS-DRG relative weights for FY 2022.
In evaluating MS-DRG changes and setting MS-DRG relative weights, CMS typically relies on claims data captured in the MedPAR file and cost report data captured in the Healthcare Cost Report Information System file. In a traditional year, for ratesetting purposes, CMS uses the most recent data available at the time of rulemaking, which normally captures claims from discharges that occurred for the fiscal year two years prior to the fiscal year addressed in the rulemaking. For FY 2022, therefore, CMS normally would analyze data from FY 2020.
In light of the PHE, however, CMS believes that FY 2020 data would not accurately reflect utilization patterns, and therefore finalized its proposal to use FY 2019 MedPAR claims data in setting inpatient hospital payment rates for FY 2022. mcdermottplus.com
New Technology Add-On Payments
Cost Criterion for NTAP
Key Takeaway: CMS finalized its proposal to use FY 2019 MedPAR data for establishing proposed FY 2023 threshold values.
Under the NTAP program, CMS provides additional payment for new medical services or technologies where the costs of the technology are not yet reflected in the MS-DRG weights. One criterion for assessing whether a new technology qualifies for an add-on payment is whether the charges for the technology meet or exceed certain threshold amounts. Historically, CMS has evaluated this cost criterion using threshold amounts established in the prior year’s final rule.
Because of the pandemic’s impact on utilization, CMS finalized its policy as proposed to use FY 2019 MedPAR data instead of FY 2020 MedPAR data. CMS made no changes to the other criteria it considers when evaluating a new technology’s eligibility for add-on payments (i.e., newness and substantial clinical improvement).
Extension for Technologies with Expiring NTAP Period
Key Takeaway: CMS finalized a one-year extension for technologies whose NTAP period was scheduled to expire.
The NTAP period normally includes the first two to three years that the product is on the market, after which the costs are captured in the MS-DRG weights. CMS evaluates the eligibility of new technologies for this additional payment annually based on their newness date (typically defined as the date of market entry). Under current policy, CMS only extends add-on payments for an additional year if the three-year anniversary for the newness date occurs in the latter half of the upcoming fiscal year.
As noted, CMS finalized its proposal to use FY 2019 MedPAR data for the FY 2022 rate-setting process for IPPS, rather than the FY 2020 MedPAR data. Because the FY 2019 MedPAR data may not fully reflect the costs of new technologies with expiring NTAP periods, CMS implemented a one-time NTAP extension for new technologies with expiring NTAP periods. This policy applies to 13 technologies and services.
The one-time extension of the NTAP period is also consistent with the proposed extension of the transitional pass-through period in the hospital outpatient setting.
NTAP Applications for FY 2022
Key Takeaway: CMS continues to see growth in the number of NTAP applications.
In the proposed rule, CMS discussed 37 NTAP applications. Following the withdrawal of some applications, CMS rendered decisions on 31 NTAP applications. Of these, 18 devices and drugs applied through the traditional pathway, and 13 went through alternative pathways (10 devices with breakthrough status and three products designated as qualified infectious disease products). Of the 31 applications discussed in the final rule, CMS approved 17 new services and technologies for the add-on payment.
There will be 40 technologies and services eligible for NTAP in FY 2022, taking into consideration the 13 new mcdermottplus.com 5 technologies that received a one-time extension of their NTAP period, 10 new technologies that were approved in FY 2021 and will still be in their NTAP period in FY 2022, and the 17 newly approved technologies.
In response to the PHE and in light of the development of new drugs and biologics for COVID-19 treatment, CMS established a new COVID-19 treatment add-on payment (NCTAP), starting with discharges on or after November 2, 2020, that met certain criteria. Acknowledging the pandemic’s continued financial impact on hospitals, CMS finalized its policy to continue the NCTAP for qualified technologies that do not qualify for the NTAP. The NCTAP remains in effect until the end of the fiscal year following the end of the PHE.








 />i
/>i


